We draw blood and measure the Coenzyme Q10 concentration in either plasma or serum at baseline and again after so many hours or days of supplementation.
What is it that makes an oral medication effective? To be effective, a drug or dietary supplement or herbal remedy has to be absorbed, and it has to become available for the body to use in the relevant cells and tissues.
Q10 and Chronic heart failure
For example, in chronic heart failure patients, the orally ingested ubiquinone Q10 has to be absorbed in the small intestine and has to be transported by the lymphatic system to the venous blood and then through the right heart and through the lung where oxygen is added to the blood. From the lung, the blood goes to the left heart and is then pumped throughout the body to all cells and tissues. Thus, the Q10 reaches the heart, the brain, and the other vital organs.
Q10 = a big molecule to be absorbed
The thing is, Q10 is a fairly big molecule to be absorbed. The Q10 molecules are obtained from the manufacturer in a crystalline form because of the purification process, and these Q10 crystals cannot be absorbed in the small intestine. In the body itself, the Q10 has to be in the form of single molecules and not in the form of crystals. The producer and seller of a Q10 preparation must succeed in breaking the Q10 crystals down into single molecules because intestinal absorption takes place on a molecular level.
Q10 must not re-crystallize inside the capsule
The manufacturer must dissolve the single Q10 molecules in an oil solution and must encase the single Q10 molecules in capsules that protect against the harmful effects of light. The important thing is that the Q10 molecules should not re-crystallize inside the capsule. Without a good stabilizing molecule to prevent recrystallization from occurring, there is a danger that the Q10 will do just that.
Claims for 10 absorption
Some producers of Q10 preparations make claims of High Absorption or Superior Absorption or Improved Absorption to describe the absorption or the bioavailability of their product. If a company claims 100 percent absorbable, the consumer should be wary. There are not any Q10 products that give 100 per cent absorption. And claims of better or improved absorption are just as suspicious, especially if the “improved” product is being compared to a dry powder Q10 product instead of being compared to a lipid-based Q10 product.
Q10 absorbed in a passive process
From Dr. William Judy, I learned that Q10 is absorbed by a process called “simple passive facilitated diffusion.” He explained that “passive” means that the process does not require any active use of energy to effect the absorption and that “facilitated” means that there must be lipid carrier molecules present to “carry” the Q10 molecules through the absorption process.
Q10 absorption and transport
To recapitulate, absorption is what passes through the absorption cells into the lymph and then into the blood. The peak absorption comes normally 5 to 8 hours after ingestion. The half-life of the Q10 in the body is about 33 hours (1).
Q10 absorption leads to Q10 bioavailability
Bioavailability, as distinct from absorption, is the accumulation (storage) of Q10 in the blood. Bioavailability is measured at 7, 14, 21, and 30 days of a set daily dosage.
If no Q10 is absorbed, then, obviously, none can be accumulated in the blood and be available for cellular uptake. Put another way, absorption leads to bioavailability.
How to study Q10 absorption
If the same participants are used, then Q10 absorption studies need to be completed before any bioavailability studies are started because the absorption curve will vary considerably depending on the blood and cellular Q10 levels at the time of the absorption study.
For a good absorption study, the participants should have fasted and rested overnight. They should not have eaten any food containing Q10 before and during the absorption study, and the participants should not engage in any strenuous physical activity.
Measuring Q10 increases resulting from supplementation
The concentrations of Q10 in plasma or serum at baseline and then again after a period of supplementation are measured and reported in either micrograms of Q10 per milliliter or milligrams of Q10 per liter.
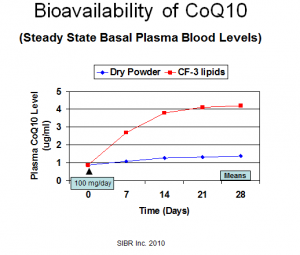
The researchers at Dr. Judy’s SIBR Research Institute have seen Q10 absorption ranging from 0.2 to 2.2 ug/ml. That is to say, they have seen Q10 supplementation increase the plasma Q10 concentrations by those amounts. The researchers have seen a range of 30-day bioavailability from 1.3 to 4.2 ug/ml.
Coenzyme Q10 concentrations in plasma and in serum will vary considerably according to the following factors:
- Age (there is lower Q10 bio-synthesis with increasing age)
- Diet (meat and fish versus little or no meat and fish)
- Disease conditions (decreased Q10 concentrations observed in people with cancer, diabetes, heart disease)
- Exercise regimes (strenuous endurance exercise in particular)
- Medication (statin medications have been shown to reduce the bio-synthesis of Q10)
Basically, in the United States, we might expect that adults would have baseline blood Q10 levels ranging between 0.43 and 1.53 milligrams per liter. Then, depending on the dosage and the timing of the dosage – with or without a meal, once or twice daily — , regular supplementation with a well-absorbed Q10 supplement should raise the blood Q10 level by a full milligram per liter and perhaps a little more. But there is much variability from person to person and situation to situation.
Dr. Judy’s summing up
What have Dr. Judy and the SIBR Research Institute researchers learned over the years?
- Not all Q10 product types are absorbed equally well.
- Crystalline Q10 products are poorly absorbed.
- Crystal-free products with facilitating lipid carrier are absorbed best.
- The human body cannot absorb a crystal.
- Only single Q10 molecules can be absorbed.
- Very little of the crystalline Q10 will dissolve in a lipid at body temperature without some sort of special heat pre-treatment.
- Single Q10 molecules without a facilitating lipid are poorly absorbed.
- There is always the danger that the Q10 product – especially the ubiquinol products — will re-crystallize inside capsules.
Documented effects of Q10 supplementation
Coenzyme Q10 products have to be prepared carefully if the ubiquinone Q10 is going to be absorbed. Claims for absorption need to be documented in reports in peer-reviewed journal articles. You should be able to get journal article citations from the Q10 producers who have documented the absorption of their products.
Documentation of absorption is a necessary condition before I will purchase a Q10 product. But is it, in and of itself, a sufficient condition? I would like to see more. I would like to see documentation of the effect of taking the product. And that is what we have in the documented effects of taking Q10 supplements in the Q-symbio study (2) and in the KiSel-10 study (3) and in the Gulf War Illness study (4). We see documented effects such as the following effects:
- Fewer major adverse cardiovascular events
- Fewer cardiovascular deaths
- Fewer all-cause deaths
- Lower incidence of hospital stays for Heart Failure
- Improvement of New York Heart Association classification
Sources:
- Bhagavan, HN, Chopra RK. (2006, May). Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radical Research, 40(5):445-453.
- Mortensen SA, Rosenfeldt F, Kumar A, et al. The Effect of Coenzyme Q10 on Morbidity and Mortality in Chronic Heart Failure: Results From Q-SYMBIO: A Randomized Double-Blind Trial. JCHF. 2014;():. doi:10.1016/j.jchf.2014.06.008.
- Alehagen, U., Johansson, P., Björnstedt, M., Rosén, A., & Dahlström, U. (2013). Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. International Journal Of Cardiology, 167(5), 1860-1866. doi:10.1016/j.ijcard.2012.04.156
- Golomb, B. CoQ10 and gulf war illness. Neural Computation 2014 Nov; Vol. 26 (11), pp. 2594-651





Leave A Comment